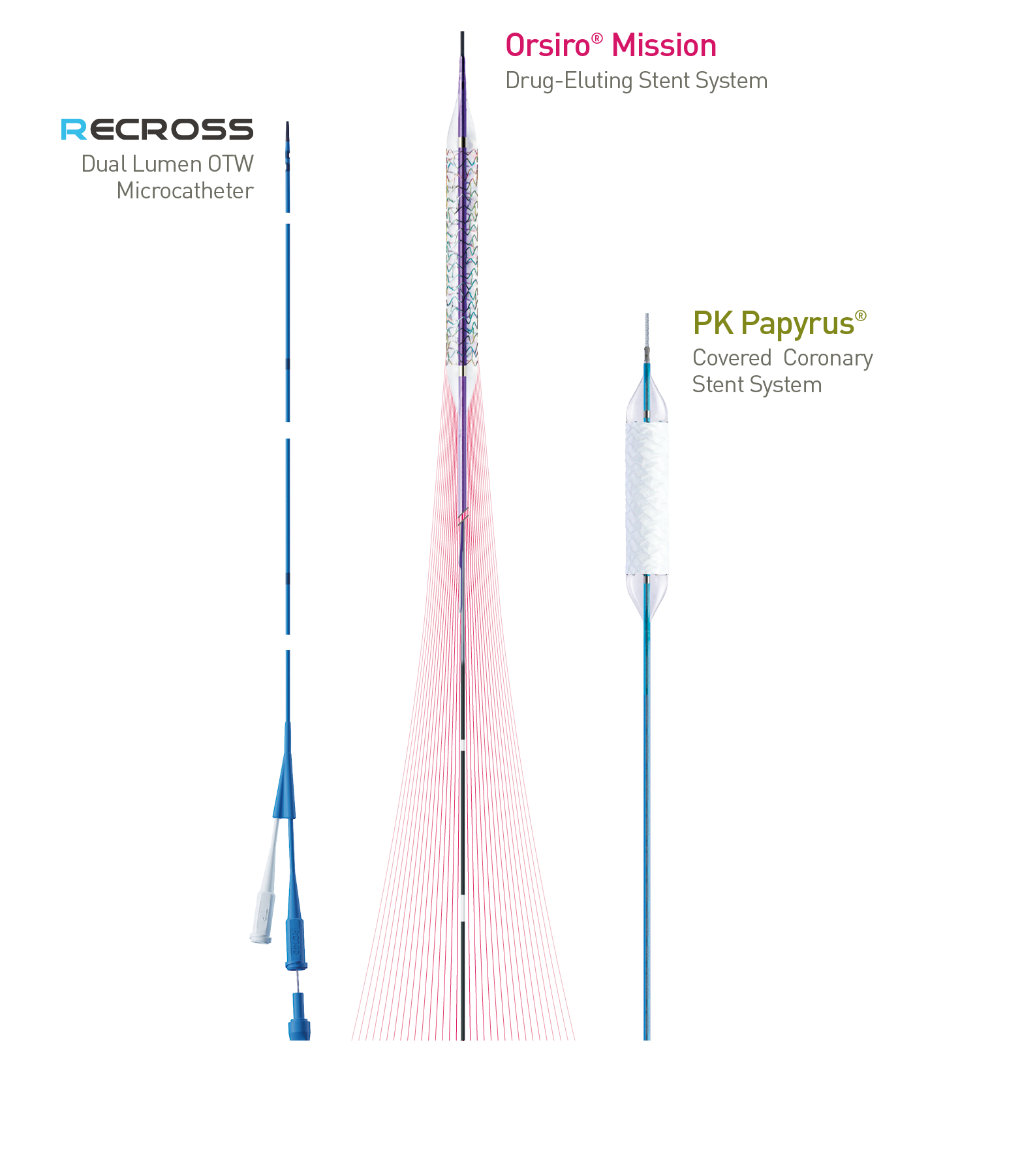
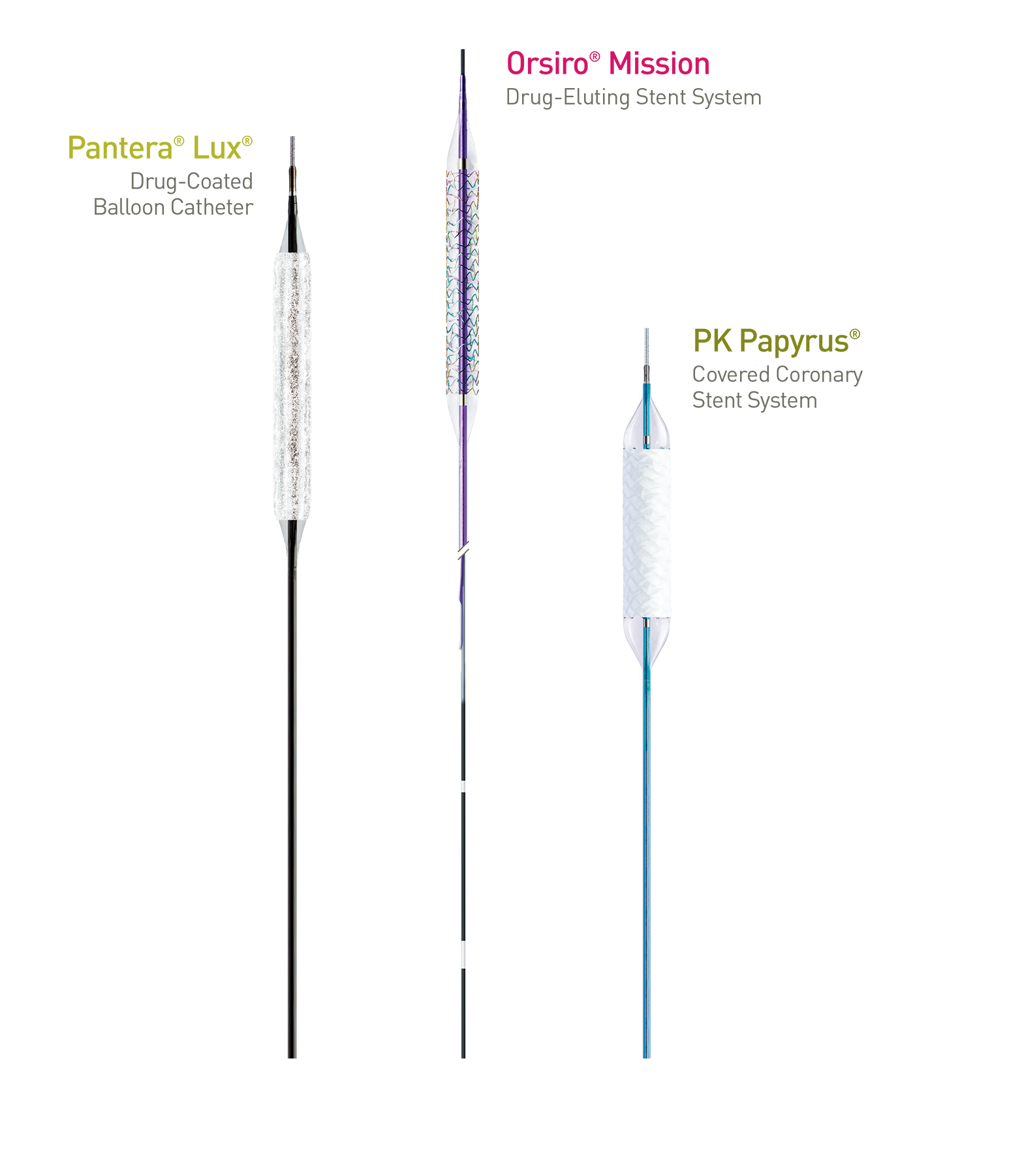
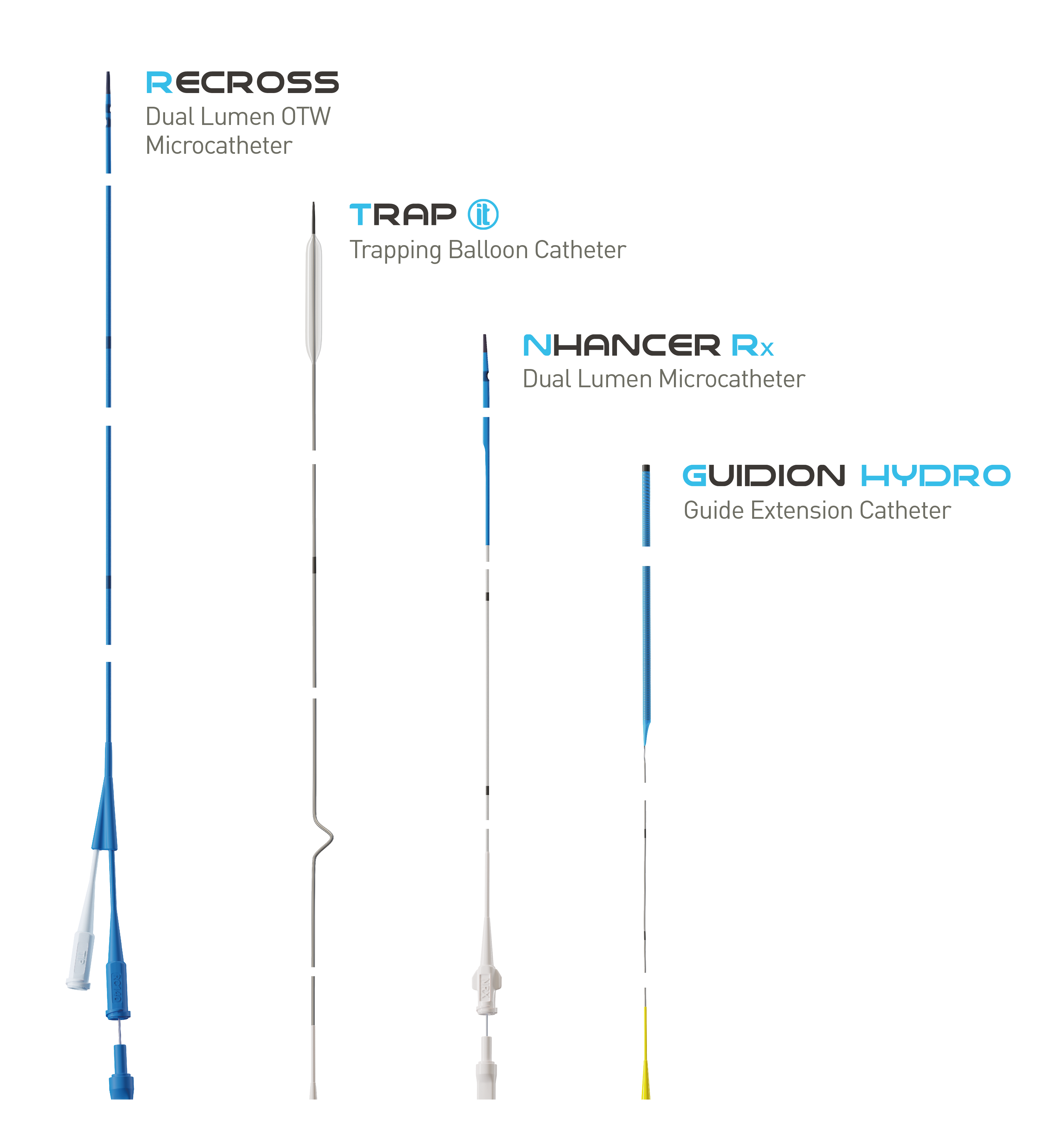
Treat the Complex



Get in touch to learn more
Please fill out the form below and a BIOTRONIK representative will contact you shortly. Thank you for getting in touch.
1.In comparison to Xience Sierra, Resolute Onyx and Synergy for bench tests on pushability, trackability, and crossability, BIOTRONIK data on file; 2. As characterized with respect to strut thickness in Bangalore et al. Meta-analysis; 3. Based on investigator’s interpretation of BIOFLOW-V primary endpoint result; 4. Tölg R et al. Coronary artery treatment with paclitaxel-coated balloon using a BTHC excipient: clinical results of the international real-world DELUX registry EuroIntervention. 2014; 10(5). 591 -599; 5. Hehrlein C et al. Twelve-month results of a Paclitaxel Releasing Balloon in Patients Presenting with In-stent Restenosis First-in-Man (PEPPER) trial. Cardiovascular Revascularization Medicine. 2012; 13 (5): 260-264; 6. Kufner S, Joner M, Schneider S et al. Neointimal Modification With Scoring Balloon and Efficacy of Drug-Coated Balloon Therapy in Patients With Restenosis in Drug-Eluting Coronary Stents. JACC Cardiovasc Interv. 2017; 10(13). 1332 -1340; 7. Jensen C et al. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: the BIOLUX randomised controlled trial. EuroIntervention. 2018; 14: 1096-1103; 8. Nguyen V.P.T et al. Comparison of clinical outcomes of two different types of paclitaxel-coated balloons for treatment of patients with coronary in-stent restenosis. Heart and Vessels. 2019. 1-9. doi: 10.1007/s00380-019-01388; 9. Assadi-Schmidt A et al. SeQuent Please vs. Pantera Lux drug coated balloon angioplasty in real life: Results from the Düsseldorf DCB registry, Int J Cardiol. 2016. doi: 10.1016/j.ijcard.2016.12.022; 10. Vos NS et al. Safety and feasibility of a PAclitaxel-eluting balloon angioplasty in Primary Percutaneous coronary intervention in Amsterdam (PAPPA): one-year clinical outcome of a pilot study. EuroIntervention. 2014; 10(5). 584 -590; 11. Vos N S et al. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction (The REVELATION Randomized Trial). JACC: Cardiovascular Interventions. 2019; 1-9, doi:10.1016/j.jcin.2019.04.016; 12. Worthley S, Hendriks R, Worthley M et al. Paclitaxel-eluting balloon and everolimus-eluting stent for provisional stenting of coronary bifurcations: 12-months results of the multicenter BIOLUX-I study. Cardiovasc Revasc. Med. 2015; 16(7). 413 -417; 13. Jim MH et al. Six month angiographic result of supplementary paclitaxel-eluting balloon deployment to treat side branch ostium narrowing (SARPEDON). Int J Cardiol. 2015; 187:594 -597; 14. Roncalli J et al. Paclitaxel Drug-Coated Balloon After Bare-Metal Stent Implantation, an Alternative Treatment to Drug-Eluting Stent in High Bleeding Risk Patients (The Panelux Trial). J INVASIVE CARDIOL. 2019;31(4):94-100; 15. García-Touchard A, Goicolea J, Sabaté M et al. A randomised trial of paclitaxel-eluting balloon after bare metal stent implantation vs. bare metal stent in ST-elevation myocardial infarction (the PEBSI study). EuroIntervention. 2017; 12(13). 1587 -1594; 16. Radke PW et al. Vascular effects of paclitaxel following drug-eluting balloon angioplasty in a porcine coronary model: the importance of excipients. EuroIntervention. 2011 Oct; 7(6): 730-7; 17. BIOTRONIK data on file; 18. Compared to Graftmaster 2.8/16, BIOTRONIK data on file; 19. IMDS data on file.
Orsiro Mission, PK Papyrus, Pantera and Lux are trademarks or registered trademarks of the BIOTRONIK Group of Companies.
Clinical data collected with the Orsiro DES and/or Orsiro Mission DES device within the Orsiro family clinical program.
NHancer Rx, TrapIT and Guidion Hydro are trademarks (ReCross a product) of IMDS. Distributed by BIOTRONIK in selected countries.